Differential Pressure Sensor Calibration: The Unseen Gatekeeper of Cleanroom Integrity
In industries that demand strict environmental control—such as pharmaceuticals, semiconductors, biotechnology, and healthcare—the Clean Room serves as the most critical production area. However, the “cleanliness” of this room comes not from basic cleaning but from the absolute control of airflow and pressure. Within this complex control system, the Differential Pressure Sensor (DPS) is the pivotal device. It is more than a measuring tool; it is the unseen gatekeeper, constantly monitoring and protecting the physical barrier against contamination. If this sensor drifts out of tolerance, the entire production process and product quality face a major risk. Therefore, DPS calibration is not merely a technical task, but a mandatory quality and legal requirement to maintain the cleanroom’s integrity.
1. Core Application: Why Differential Pressure is the Lifeblood of a Cleanroom

Differential pressure measures the difference in air pressure between two adjacent areas, typically measured in Pascals (Pa) or inches of water (inH2O).
CONTAMINATION CONTROL
Maintains positive pressure to prevent contaminated air ingress.
HEPA/ULPA FILTER MONITORING
Measures pressure drop across filters to detect clogging and maintain airflow
PRODUCT SAFETY
Essential for pharmaceutical, medical, and microelectronics quality.
1.1. Securing the Contamination Barrier (Positive Pressure)
The primary use of a DPS in a cleanroom is to maintain and monitor Positive Pressure.
- Protecting the Clean Zone: In cleanrooms producing pharmaceuticals or microchips, the internal pressure is always maintained higher than the surrounding environment (typically to ). The DPS accurately measures this difference. When a door opens, air will always flow out, effectively creating an invisible air barrier. This crucial mechanism prevents dirt, microorganisms, and contaminants from the less-clean area from being drawn into the clean zone.
- Controlling Containment (Negative Pressure): Conversely, in high-level biosafety laboratories (BSL-3, BSL-4) or infectious patient isolation rooms, the DPS must ensure Negative Pressure. This guarantees that air and dangerous pathogens are always contained inside and cannot leak to the outside environment.
1.2. Monitoring HEPA/ULPA Filter Blockage
HEPA/ULPA filters are the heart of the cleanroom’s HVAC system; they remove over 99.97% of tiny particulates.
- Clogged Filter Alert: The DPS is installed to measure the pressure difference across the filter faces. As the filter collects particulates, the pressure difference across it increases. Monitoring this reading precisely alerts technicians to the exact moment they need to replace or clean the filters, ensuring the required Air Changes per Hour (ACH) and clean airflow are maintained.
1.3. Direct Impact on Product Safety and Compliance
The stability of the differential pressure directly affects the final output quality, making it a critical Product Safety factor.
- Essential for Pharmaceutical and Medical Quality: Maintaining the correct pressure cascade is fundamental to meeting regulations like cGMP (Current Good Manufacturing Practice). If pressure fails, regulators must reject the batch produced during that time due to the high risk of contamination.
- Micro-contamination Control for Microelectronics: In semiconductor and microelectronics fabrication, even the smallest dust particles () can destroy a wafer. The DPS ensures the pressure cascade prevents particle ingress, directly protecting the delicate, high-value components.
2. The Absolute Imperative of DPS Calibration
A Differential Pressure Sensor that reads incorrectly can lead to serious and costly consequences.
COST CONTROL & FAILURE PREVENTION
Minimizes downtime and financial losses by ensuring continuous cleanroom integrity.
REGULATORY COMPLIANCE
Strictly adheres to ISO 14644 and GMP requirements.
2.1. Preventing Costly Production Failure
Imagine a scenario where the DPS reports a positive differential pressure of , but the actual pressure is only due to calibration drift. The cleanroom has lost its physical barrier.
- Contamination Risk: Microbial or particulate contamination will likely occur. This leads to Product Recall, loss of entire production batches, and massive damage to financial health and brand reputation.
- Wasted Energy: If a sensor reads too low, the HVAC system might overcompensate by working harder to push air into the room to meet the setpoint (based on the false data). This leads to significant, unnecessary energy consumption.
2.2. Ensuring Legal Compliance and Integrity
Calibrating the DPS is a legally mandatory requirement for facilities operating under key quality standards:
- ISO 14644: The international cleanroom standard requires verification of differential pressure system performance.
- cGMP (Current Good Manufacturing Practice): In pharmaceuticals and medical devices, providing evidence of continuous, controlled manufacturing environments is core. A Calibration Certificate is indispensable for passing regulatory audits by bodies like the FDA or international licensing organizations.
- Accuracy and Traceability: A calibration certificate from an ISO/IEC 17025 accredited laboratory provides documented proof. It ensures the pressure data collected is reliable and traceable back to national measurement standards.
Data and Impact of Calibration
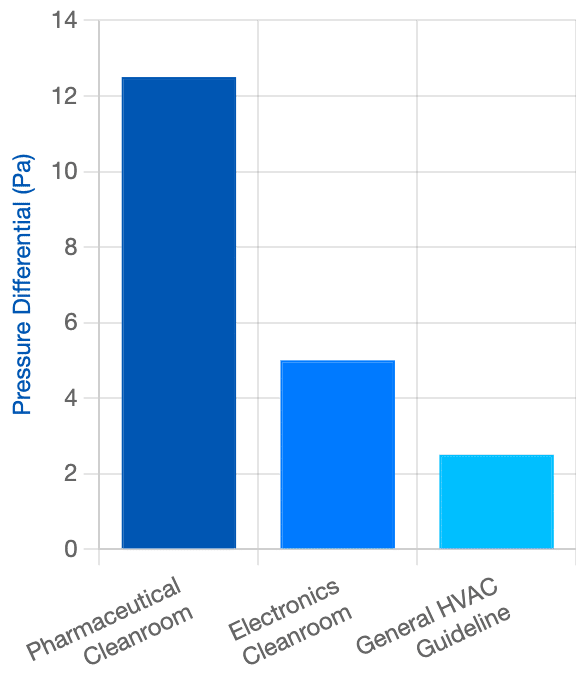
Required DPS Sensitivity
Cleanrooms require accuracy in extremely small pressure units (Pascals).
Accuracy in Pascals
Contamination Risk (1 Pa Error)
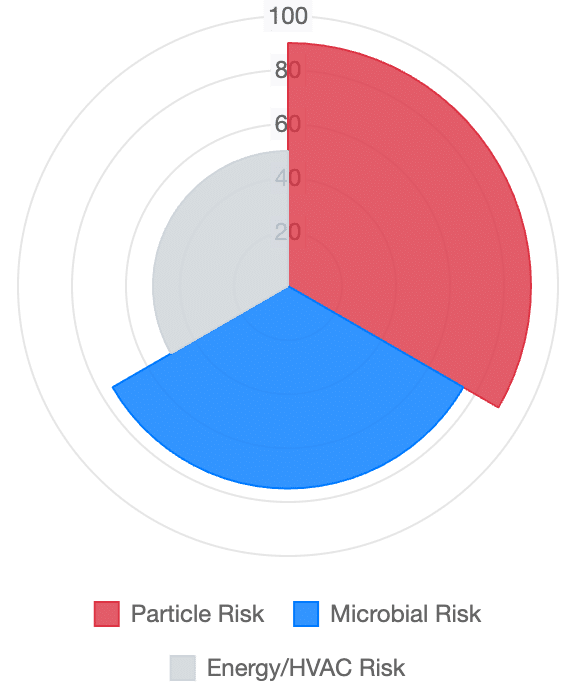
Outcomes
🟢 Benefits (Proper Calibration)
- ✅ Ensures Cleanroom Classification (ISO 5)
- ✅ Prevents Cross-Contamination
- ✅ Legal Compliance & Risk Mitigation
🔴 Risks (Calibration Failure)
- ❌ Pressure Deviation > 5 Pa
- ⚠️ Product Recall due to Contamination
- 🚨 Operational Suspension & Reputation Damage
3. The Professional Differential Pressure Calibration Process
Specialized metrology technicians must perform DPS calibration using ultra-high-accuracy reference standards.
3.1. Reference Equipment
The primary tools used are Reference Pressure Calibrators or Pressure Comparators. These devices are significantly more accurate than the sensor being calibrated.
3.2. Step-by-Step Procedure
5 Key Calibration Steps
- Initial Check: The technician visually inspects the sensor and ensures there are no leaks or blockages in the pressure tubing.
- Connection Setup: The DPS is connected to the Reference Pressure Calibrator. The technician establishes calibration setpoints (e.g., , , , ) for both positive and negative pressure ranges.
- Pressure Application: The technician applies precise, controlled pressure from the Reference Calibrator to the sensor.
- Data Recording: The technician records the actual pressure value from the Reference Standard against the value displayed on the DPS.
- Adjustment: If the error exceeds the allowed tolerance, the technician adjusts the sensor or re-configures the transmitter to bring the reading back within acceptable limits.
- Certification: A detailed Calibration Certificate is issued, documenting the environmental conditions, the reference standards used, and the “As Found” and “As Left” results.
4. Calibration Frequency
The recommended minimum frequency for DPS calibration is typically annually (12 months).
However, in highly sensitive or volatile production environments (such as aseptic processing), this frequency may be shortened to six months or even three months. Recalibration is also essential following any major repairs or component replacements within the HVAC system.
Conclusion
The Differential Pressure Sensor is a modest but critical component that safeguards billions of dollars worth of products and human safety. It is the only tool that confirms a cleanroom is truly “clean” according to required standards.
Professional, periodic calibration of the Differential Pressure Sensor is not an expense but a smart quality assurance strategy. It ensures that the physical barrier against contamination is precisely maintained, protecting the business from the most costly risks: production failure and loss of customer trust.
Have you verified the accuracy of your facility’s DPS sensors this year?



