Ion Selective Electrode Meter (ISE Meter): The Essential Tool for Environmental and Water Analysis – Why Calibration is Critical

Environmental pollution grows. Demands for water quality control increase. Consequently, fast, accurate, and reliable analytical methods become paramount. The Ion Selective Electrode Meter (ISE Meter) emerges as a leading solution. This electrochemical device empowers scientists, environmental engineers, and quality control technicians. They directly and quickly measure specific ion concentrations. This includes everything from essential nutrients to toxic pollutants. They can perform these measurements in the field or in the lab. Specifically in environmental and water analysis, the ISE Meter’s speed and in-situ capability offer significant advantages. This is true compared to more complex and time-consuming methods. However, the effectiveness and legal validity of the measured data depend entirely on one fundamental factor: Ion Selective Electrode Meter Calibration (ISE Meter Calibration).
1. Core Applications of the ISE Meter in Environmental Analysis
ISE Meters help monitor key chemical components in water, soil, and wastewater. Thus, they support regulatory compliance and ecological protection.
Core Applications of ISE
WATER QUALITY
Measures Fluoride, Chloride, Nitrate in drinking water for safety compliance.
INDUSTRIAL WASTEWATER
Controls Ammonia, Cyanide, Nitrite levels before discharge, ensuring legal compliance.
AGRICULTURAL ANALYSIS
Measures Potassium, Calcium, Magnesium concentrations in soil and plant samples.
PHARMACEUTICALS & HEALTHCARE
Ion analysis in sterile products, biological samples, and buffer solutions.
1.1. Drinking Water Quality Control
Public drinking water safety remains a top national priority. ISE Meters track ions directly affecting human health:
- Fluoride (): Controlling Fluoride concentration in tap water is crucial. Low levels do not protect dental health; high levels can cause toxicity. The ISE Meter provides fast, accurate measurements at water treatment plants.
- Nitrate (): Nitrate often originates from agricultural fertilizers or septic systems. High Nitrate levels in groundwater pose serious health concerns. Specifically, they cause methemoglobinemia (blue baby syndrome) in infants. The ISE Meter allows rapid screening of suspected water sources.
- Chloride (): We monitor Chloride levels to assess salinity. This also helps detect seawater intrusion into freshwater resources.
1.2. Wastewater Monitoring and Treatment
Wastewater treatment plants must control ion levels. This ensures effluent meets environmental standards. They do this before discharge into receiving waters.
- Ammonia/Ammonium (): These are key nutrients needing removal. High Ammonia levels harm aquatic life and cause eutrophication. The ISE Meter monitors biological reactor performance during treatment.
- Sulfide (): Sulfide often produces toxic gas. The ISE Meter helps track and control this ion’s concentration in industrial wastewater.
1.3. Soil Analysis and Agriculture
Soil quality directly impacts crop yield. The ISE Meter helps optimize resource utilization.
- Plant Nutrients: It measures concentrations of key nutrients. Examples include Potassium (), Calcium (), and Sodium (). These measurements occur in soil samples and hydroponic solutions. Farmers use this data to adjust fertilizer application precisely. This prevents waste and water source contamination.
- Salinity: The ISE Meter can measure total dissolved ion concentration in soil. This helps assess salinity, a critical factor for plant growth.
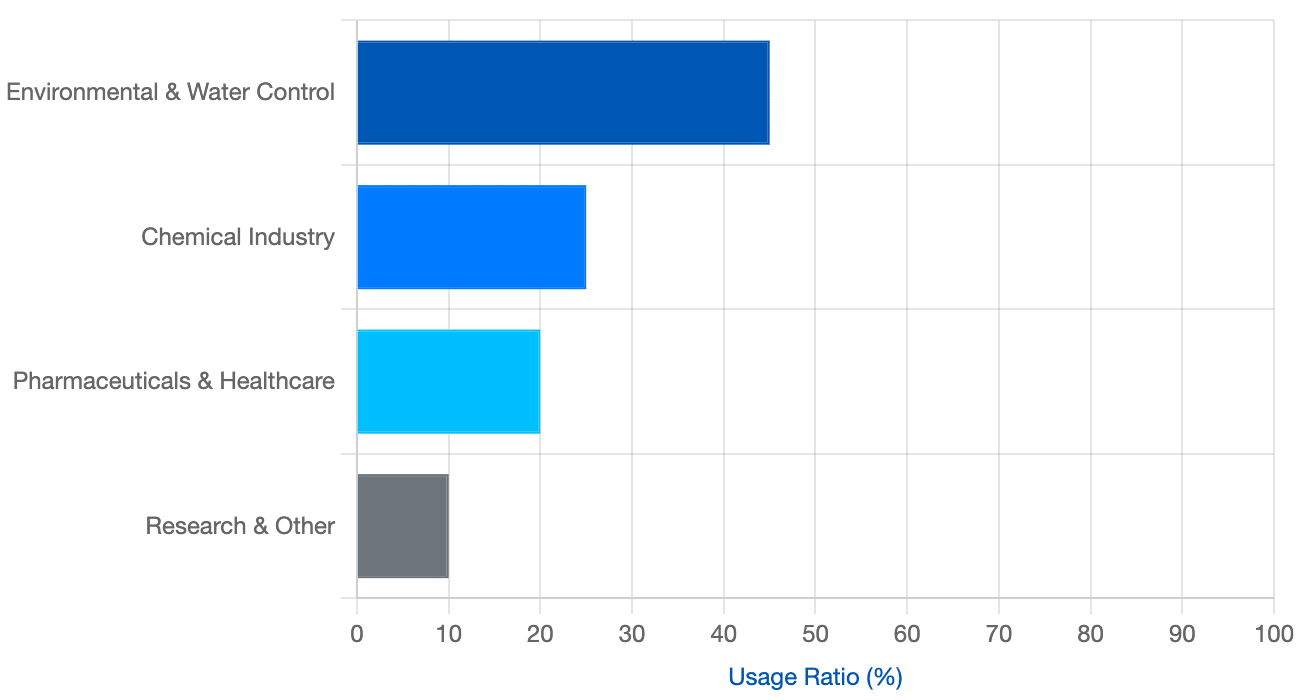
ISE Usage Distribution by Sector
The frequency of calibration needs based on common usage areas (Hypothetical Data).
Distribution of Ion Analysis Demand (%)
2. ISE Meter Calibration: The Factor Determining Data Validity
The ISE Meter offers speed benefits. Yet, it is an electrochemical device. Thus, it is highly sensitive to electrode changes. Regular calibration is mandatory. It ensures measured data holds legal and scientific value.
The Critical Role of Calibration
NERNST LINEARIZATION
Establishes the linear relationship between potential and concentration logarithm (Nernst Equation).
DRIFT COMPENSATION
Counters the change in electrode potential over time and temperature to maintain stable data.
MATRIX EFFECT ELIMINATION
Ensures interfering substances in the sample do not skew the measurement results.
2.1. Ensuring Absolute Measurement Accuracy
The ISE Meter’s ion-sensing element (electrode) works based on potential difference (voltage). This difference occurs between a measuring electrode and a reference electrode. Furthermore, the relationship between potential and ion concentration is logarithmic. The Nernst equation governs this.
- Electrode Drift: ISE electrodes wear down. Their properties change over time. Temperature shifts and usage frequency also affect them. This causes a “drift” in the calibration curve. Calibration uses Standard Solutions of known concentrations. This compensates for drift, ensuring the logarithmic relationship remains accurate.
- Slope and Detection Limit: Calibration determines the electrode’s slope. This helps the device accurately determine ion concentrations. This is true across different ranges, especially at low concentrations (the detection limit).
2.2. Fulfilling Legal and Scientific Compliance
Environmental analysis data often goes into reports for regulatory agencies. For instance, environmental protection departments use this data.
- Traceability: An Ion Selective Electrode Meter Calibration Certificate provides irrefutable evidence. This comes from an ISO/IEC 17025 accredited laboratory. It proves your device underwent proper checks and adjustments. These align with national measurement standards.
- Legal Validity of Data: Analytical results may feature in legal disputes. They might also appear in environmental compliance reports. In such cases, data must originate from a properly calibrated and maintained device. Consequently, data from uncalibrated equipment will be invalid.
Consequences of Failure (Uncalibrated ISE)
ENVIRONMENTAL FINES
Incorrectly reporting pollutant concentrations, leading to severe legal violations and fines.
PROCESS FAILURE
Deviation in pharmaceutical quality control, causing product recalls.
RESOURCE WASTE
Wasting chemicals and time by repeating unreliable analyses.
3. The Basic ISE Meter Calibration Procedure
ISE Meter calibration often needs frequent performance. Sometimes, this occurs before each use or daily. It depends on the application.
- Standard Preparation: Use at least two (or more, typically three) Ion Standard Solutions. These must have known concentrations within the desired sample measurement range. Also, these solutions must be current and accurately prepared.
- Setting Calibration Points: Place the electrode into the lowest concentration standard solution. Then, record the potential value. Afterward, move to a higher concentration solution.
- Curve Generation: The instrument automatically generates the logarithmic relationship. This occurs between the potential and the ion concentration.
- Slope Assessment: A technician checks the calibration curve’s slope. If the slope falls outside the manufacturer’s accepted range, the electrode needs replacement or maintenance.
- Accuracy Verification: After calibration, re-check the device. Use a third standard solution (not used in primary calibration). This confirms accuracy.
Conclusion
The Ion Selective Electrode Meter (ISE Meter) proves a powerful, fast, and cost-effective tool. It serves well in environmental, water, agricultural, and many other analyses. Its applications are vast. They range from ensuring safe drinking water (Fluoride, Nitrate control) to optimizing wastewater treatment (Ammonia monitoring).
However, the ISE Meter’s power relies entirely on its reliability. Regular calibration of the Ion Selective Electrode Meter is more than a technical rule. It is a key factor. It ensures scientific accuracy, the legal validity of data, and our responsibility toward the environment.



